The main benefit of nitrogen-filled tires is that the loss of tire pressure is slower, because the gas in the tire escapes more slowly than air does. With more stable tire pressure, the thinking goes, you’ll get better gas mileage and get full tire life since you’re always rolling on fully inflated tires.
Claims are also made that nitrogen in tires prevents tire “rot” by limiting the moisture that naturally occurs inside tires and heads off corrosion of the wheel that can be caused by contact with moisture.
These claims are overstated. The advantages of tires filled with nitrogen, instead of plain ol’ air, aren’t big enough to justify the price tag or the inconvenience. On new car tires, the cost can range from $70 to as much as $179. On existing tires, you’ll pay up to $30 per tire for service to drain air and refill with N2. Refills will run you $5 to $7 per tire, which you can expect to do less often than with air-filled tires.
But you’ll still need topping off every two or three months.
Small amounts of air naturally leak out of tires over time, especially when tires are subject to large temperature swings. This is because the walls of tires are slightly porous. When a tire gets hot the air inside it expands. The added pressure pushes minute quantities of air out through the pores, so you occasionally have to get your air topped off even if your tire doesn’t have a hole.
Promoters of nitrogen tires point out they don’t lose tire pressure as fast as air-filled tires. Since nitrogen molecules are bigger than normal air molecules, it is harder for them to leak out. This means a tire filled with nitrogen will maintain air pressure longer. Therefore, they say, you’ll roll on tires that are always properly inflated, resulting in better fuel economy and longer tire life.
A normal tire filled with regular air loses an average 1 to 2 PSI (pounds per square inch) per month. It’s true that there is a slower loss from nitrogen-filled tires. But this improvement is slight — only about 1.3 PSI less over the course of an entire year, according to Consumer Reports. It’s not enough to make a true difference in gas mileage or tire wear for people driving passenger vehicles.
It’s true that there is a slower loss from nitrogen-filled tires. But this improvement is slight — only about 1.3 PSI less over the course of an entire year, according to Consumer Reports. It’s not enough to make a true difference in gas mileage or tire wear for people driving passenger vehicles.
This is partly because air is already made up of 78 percent nitrogen and just under 21 percent oxygen, with the rest a mix of water vapor, carbon dioxide and other gases. When tires are filled from a nitrogen air pump this ups the percentage of N2 to between 93 and 95 percent. It’s never 100 percent.
Bottom line: Nitrogen will slow the amount of tire inflation loss to about one-third of what you’ll experience with air. This means instead of losing one to two PSI per month, you’ll lose ⅓ to ⅔ PSI per month. You’ll still need to check and top off your air roughly every other month to stay within the ideal inflation range. And you’ll spend far more than you’ll save on gas and tire tread life. You’re better off making simple tire maintenance part of your routine.
You’re better off making simple tire maintenance part of your routine.
There are more cons than pros for changing to N2 tires. For example, nitrogen filling tanks aren’t easily accessible like air compressor tanks. You’ll have to plan for refills in places that may be few and far between. This can cost you time and money. Here’s all the info.
Q. How much will it cost to get nitrogen in my tires?
A. For fills of new tires, between $70 to about $175 at some outlets. Drains of air and refills with nitrogen on current tires, up to $30 per tire. Topping off can be between $5 and $7 per tire. If you want to keep your tires within 1 PSI of the ideal, you’ll likely be topping off at least four times a year, probably more. This could be between $80 and $112 a year, and possibly a whole lot more. Compare this to paying nothing at all for regular air at a tire store, or around a buck per fill at a service station.
Topping off can be between $5 and $7 per tire. If you want to keep your tires within 1 PSI of the ideal, you’ll likely be topping off at least four times a year, probably more. This could be between $80 and $112 a year, and possibly a whole lot more. Compare this to paying nothing at all for regular air at a tire store, or around a buck per fill at a service station.
Q. Are they safe?
A. They’re as safe as regular tires. Nitrogen isn’t flammable and won’t cause your tires to explode.
Q. Will I get better gas mileage?
A. You’ll always get better fuel economy on properly inflated tires, whether they’re filled with nitrogen or air. Under-inflated tires can lower gas mileage by about 0.2% for every 1 PSI drop in the average pressure of all tires. They’ll also wear faster and be more prone to failure. The most economical way to make sure you’re driving on well-inflated tires is to just check your tire pressure once a month or get it done by a technician (free at good tire stores).
Q. Will nitrogen prevent tire rot? Wheel rust?
A. Nitrogen is a “dry” gas compared to oxygen (which makes up about one-fifth of regular air). Nitrogen-filled tires don’t generate as much moisture inside when tires expand from heat friction then contract when they cool.
However, rubber rot from moisture inside the tires of passenger vehicles is very unusual. Unless your tires are on a vehicle that’s rarely driven, it’s far more likely your tire tread will wear out before the small amount of moisture inside an air-filled tire degrades the rubber.
And today’s alloy wheels are coated to prevent corrosion on steel parts — the belts, beads and sidewall buttressing — that may come into contact with water, so that’s not a typical problem.
Q. Can nitrogen tires be filled with air?
A. Yes. It’s unsafe to drive around on under-inflated tires, so don’t hold off thinking you need to wait to top off until you can get to a filling tank. It’s perfectly fine to add air and just get your next fill with nitrogen.
It’s perfectly fine to add air and just get your next fill with nitrogen.
Q. Do they run cooler?
A. There’s no significant difference between air-filled and nitrogen-filled tires in terms of running temperature.
Q. Where can I fill my tires?
A. Use this nitrogen dealer locator, but be aware that some filling stations require you to have purchased tires with them, or have a membership.
Q. Will I have a better ride?
A. There’s no difference in handling or ride quality between tires filled with air or nitrogen, so long as they’re kept properly inflated.
Q. How can I tell if I have nitrogen in my tires?
A. The tire valve stem will have a green plastic cap or a cap topped with a green indicator.
Q. How do tires get filled with nitrogen for the first time?
A. The tire is purged of air and filled with nitrogen several times using a machine, which takes out most of the oxygen along with any water.
Learn More
Tips for selling Nitrogen Tire Inflation to your customers.
Before you start selling Nitrogen Tire Inflation to your customers, keep the following in mind:
Our suggestion is to price the service between $8 and $12 per tire when converting an air-filled tire to a Nitrogen-filled tire, depending on the size of the vehicle’s tires. From that point on, perform free Nitrogen top-off’s as required.
We also suggest getting creative with your marketing and pricing. For example:
If selling the tire also, offer Nitrogen at a reduced cost, or include it in the price of the tire.
Include Nitrogen inflation as part of a tire service package, such as tire rotations, balances and alignments.
If you’re a new car dealer, add $50 to the sticker price of the vehicle.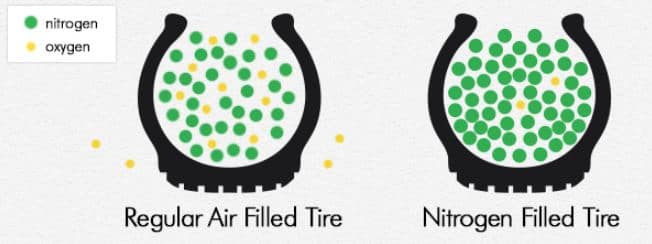
Don’t forget the spare tire! To most customers, the spare tire is "out of sight, out of mind" but they will appreciate a properly inflated spare when they need it.
We have found that the above pricing strategies work no matter your geographic location. Customers are willing to pay for peace of mind and to see savings in their pocketbook!
It’s time to sell the service! Actually, there really isn’t much selling involved at all. Nitrogen tire inflation sells itself. Here’s the basic process we recommend:
If it did, and your shop didn’t do the original Nitrogen inflation, check the purity of the Nitrogen in the tires. If it’s less than 96%, you have a sales opportunity, since Nitrogen purity of less than 96% provides diminished benefits.
Document the pressure reading of each tire, along with the recommended pressure on the vehicles door jamb placard.
It’s likely that the customer’s actual tire pressures are below (in many cases, significantly below) the recommendation provided by the vehicle manufacturer. In this case, explain the benefits of Nitrogen tire inflation and the low per-tire cost involved, and you will find that a large number of customers will purchase the service.
If the tire pressures match the vehicle manufacturer specifications, congratulate the customer on properly maintaining their tires—it’s a rarity!
Remind the customer that inflating with Nitrogen does not mean they no longer need to check tire pressures. Welcome them back to your shop at any time to top off their tires with Nitrogen for free. Not only is this a benefit to your customer but over time you are building a trust relationship with them.
Also mention to them that if they are out of town on a road trip and notice a tire that is low on pressure, it’s perfectly fine to top the tire off with regular air to make the tire safe again. Encourage them to bring the vehicle to you as soon as they return home so that you can find out why the tire is losing pressure (for example, a puncture).
Encourage them to bring the vehicle to you as soon as they return home so that you can find out why the tire is losing pressure (for example, a puncture).
Autopro Distributing, Inc
6026 Kalamazoo Ave SE
Suite 121
Kentwood, MI 49508
Phone: 616.282.1360
Text: 616.282.1360
Contact Us
Copyright 2022 Autopro Distributing, Inc.
Terms of Service | Privacy Policy
Grand Rapids Website Design by Visioneer Consulting
What is better - free air or "magic" nitrogen in tires? There are a lot of opinions. Those who pumped nitrogen into tires instead of air recommend that their acquaintances and friends do the same. Many have heard that Formula 1 cars use nitrogen to inflate tires. Yes that there "Formula 1"! Aircraft tires, heavy trucks and supercars also contain nitrogen. Opinions were divided.
Opinions were divided.
What are the advantages of nitrogen over oxygen in tires, is there any difference at all, or is it a banal pumping out of money? “Sellers of air” name such pluses:
| - stable pressure in tires, as a result of which wear is reduced; - smooth running of the car; - good road grip; - in the event of a tire puncture, the leakage rate is less; - regardless of the temperature in the tire constant pressure; - good fuel economy. |
|
At first glance, for little money, how many useful and important properties at once! Modern car owners love all sorts of exotic things, like miracle powders, wiper spoilers that supposedly improve aerodynamics, etc. They also seized on this "innovation" with nitrogen in tires.
If you recall the physics from the school course, it is clear that "air" consists of 78% nitrogen, 21% oxygen, 1% carbon dioxide and other gases. And the mixture advertised by tire fitters consists of 95% nitrogen and 5% oxygen.
And the mixture advertised by tire fitters consists of 95% nitrogen and 5% oxygen.
« Stable tire pressure. Since the coefficient of thermal expansion of nitrogen is lower than that of air, the effect of ambient temperature on the tire has practically no effect on the pressure inside it. Nitrogen does not expand at all, unlike air. Therefore, it is nitrogen that is ideal for pumping into tires. »
| However, anyone with even a little knowledge of physics understands that the statement that the pressure of a gas in an enclosed space is independent of temperature conflicts with the laws of Gay-Lussac (for any gases, the volume expansion coefficient is the same) and Charles (the pressure ratio to temperature is a constant value). We can conclude: all statements that nitrogen will behave differently than oxygen, with an increase or decrease in temperature, are real inventions that are designed for an uneducated person. |
Accordingly, the change in tire pressure will be about 0.00025 atm. Is this a significant change? Certainly not. For those who do not believe in science, we can advise you to conduct a small experiment on your own: pump one tire with nitrogen, and the other with air, and alternately immerse it in boiling water, then in ice water. It is unlikely that the pressure will be stable.
« A tire filled with nitrogen never deflates. Nitrogen molecules are very large, much larger than oxygen, and they move extremely slowly through the micropores in rubber. »
| Again we turn to physics. The size of a nitrogen molecule is 0.364 nm, and an oxygen molecule is 0.346 nm. This difference is not perceptible by any manometer. |
Perhaps the whole secret is that “large” nitrogen molecules seem to clog the micropores of the tire and do not allow molecules of other gases to pass out? Although the mixture advertised by the sellers contains 16-17% more nitrogen than ordinary air
« The possibility of tire explosion is minimal. Because nitrogen is an inert gas and does not support combustion. At high speeds, the tire does not heat up because there is no combustible oxygen in it. »
| So, let's try to understand all this. If you look at the periodic table, you can immediately see that inert gases are in group 8, and nitrogen belongs to group 5.
|
A normal tire for a passenger car can withstand pressure up to 9atm. In order for a tire to burst, it must be heated to a temperature of at least 1000 ° C. At this temperature, even a steel disk will melt.
« Fuel economy. A wheel filled with nitrogen is lighter in weight than a wheel filled with air. Accordingly, the load on the suspension is less and fuel consumption is reduced. »
| At first glance, everything is logical. But let's calculate what is the difference in the mass of wheels pumped up with nitrogen and air. 1 cubic meter of air contains 78% nitrogen - this is 1.29kg, and pure nitrogen - 1.25 kg. For example, let's take a common wheel with a 165 / 70R13 tire and calculate the mass of gas in it. |
This means that the nitrogen content in this tire will be 0.0750 kg, and air - 0.0774 kg. That's the whole difference! You just need a jewelry scale to catch such a difference in weight. Naturally, there can be no talk of any difference in weight and fuel economy.
« Delayed tire aging due to the absence of dust, moisture and oil in nitrogen. This is confirmed by tests conducted by Continental, Bridgestone, Michelin. »
| If you think about it, the impact of the environment (various reagents on the road surface, ultraviolet radiation, bitumen, etc.) on the tire is much larger than the impact of the internal filler.
|
Is it really possible to save the carcass of tires from oxidation by ordering nitrogen into the tire, as the “air sellers” promise? This is hard to believe, since it is well hidden in the thickness of the rubber and cannot come into contact with air, moreover, the wires of the frame are covered with brass and are not easily oxidized.
« Improving road grip. Nitrogen is more stable than air (which is able to succumb to the environment) . »
| This myth is generally difficult to somehow comment on. There is nothing to discuss, no matter how you look at it. Anything (the condition of the road itself, the design of the tire, the quality of the rubber from which the tire is made, the distribution of stress in the contact patch) affects the grip of tires with the road surface, but not the gas that is pumped into this tire.
|
On the other hand, cunning sellers sometimes deliberately underfill the tires with nitrogen and warn the client not to inflate the tires with air in any case, and also not to check the pressure.
So nitrogen in tires, instead of ordinary air, is not an innovation at all, but rather a tribute to fashion, which is usually not consulted with science. On the other hand, the small money that is given to the “air sellers” for nitrogen may well be compensated by the impression made on friends when saying the phrase: “And in my car there are nitrogen tires, like Schumacher’s!”.
From time to time, motorists are faced with a proposal to fill tires with nitrogen instead of air. Service sellers promise a whole host of benefits - temperature stability, wheel weight reduction, explosion safety, lower descent speed. And so on and so forth... Let's see if this is true, or just a scam calculated on ignorance. The basic school physics course will help us with this.
The first thing a car enthusiast needs to know is that air is 78% nitrogen. Nitrogen can be pumped into tires with a maximum proportion of 95%. That is, we are talking about a difference of only 17% of the total volume of injected gas. It is from these figures that we must proceed.
RUBBER WEAR
One of the main claims is that the tire wears less due to the fact that nitrogen is less subject to thermal expansion. This statement is false because Gay-Lussac's law states that the change in volume of a gas with temperature is directly proportional. That is, no matter what gas is pumped into the tires, the pressure will change the same way. Only 3 factors affect tire wear: rubber compound, mileage and driving style.
BETTER GRIP AND MANAGEMENT
It doesn't even occur to me how this assertion can be substantiated. Grip with the surface depends solely on the properties of the tire itself and the tread pattern. With the correct pressure in the wheel, it does not matter at all what gas is there. This is a false statement.
TIRES LOSE PRESSURE SLOWER
This statement is based on the fact that the nitrogen molecule is larger than the oxygen molecule and therefore passes through the tire body more slowly. This is partly true. The size of a nitrogen molecule is 3.1x10 to the -8th power, while an oxygen molecule is 2.9x10 to -8 power. The difference is only 6.5%. Considering that we are talking about only 17% of the difference, we find that the rate of pressure loss in a tire with ordinary air will be only 1.1%. That is, under ideal conditions, the tire pressure will become slightly less in at least a few years. Tires just don't last that long. In general, pressure loss is usually caused by a faulty nipple or hole punching when the sidewall of the tire moves away from the rim for a short time.
WHEELS GET LIGHTER
This statement is partly true. But let's count how much. The mass of a nitrogen molecule is 14 units, oxygen - 16 units. The difference is 14%. That is, the mass of air in a tire with a pressure of 2.2 atmospheres will be 0.14x0.17x2.2 = 0.05, that is, about 5%. And this is not the mass of the wheel, but only the mass of air in it. In fact, you cannot measure the difference in weight even on a scale. Is it worth paying money for it?
REDUCED TIRE EXPLOSION BECAUSE NITROGEN IS AN INERT GAS AND DOES NOT SUPPORT COMBUSTION
First, nitrogen is not an inert gas. The inert gases we have on Earth are only helium, neon, argon, krypton, xenon and radon. Secondly, tires do not explode, but burst due to a difference in pressure. In this case, there is no ignition and cannot be. And any tire can burst, no matter what it is inflated with.
TIRE AND DISC CORROSION PREVENTION
Another partially true statement. Yes, oxygen causes oxidation, but the extent of its effect on painted rims and tires is so small that we are talking about decades. At the same time, the cost of regular nitrogen injection will more than cover the cost of both.
TIRES ARE LESS DEFORMABLE AND INCREASED COMFORT DUE TO SOFTNESS
These two statements absolutely contradict each other. Comfort when moving directly depends on the pressure in the wheel. The lower the pressure, the softer the tire, because it deforms more when it hits an obstacle. How nitrogen affects the ability to deform, no one knows. There are times when, in order to create the appearance of increased comfort, nitrogen is simply not pumped in, thereby making the tires softer. A person travels and does not even suspect that he has been “divorced”.
NITROGEN IS PUMPED INTO RACING CARS
This argument is very often given, referring to Formula 1 racing, but frankly disingenuous.