Today, you can buy tires that can carry your vehicle tens of thousands of miles. Modern tires are the results of decades of tire engineering progress. The tire compound is a versatile material that comes with a range of properties depending on the raw materials used to manufacture it.
These materials allow tire manufacturers to come up with detailed tire designs that stand the test of time and driving pressure. But what are tires made of? How to make tires that are actually durable in the long run?
There are some important physical and chemical properties at play that make vehicle tires safe for everyday use. One of the main feedstocks required in tire making is crude oil. Below, we are going to delve a bit into the tire manufacturing process.
We are going to show you how much oil they need to produce a single tire and why. For this, we need to delve a bit into rubber production as well.
What are Car Tires Made of Exactly?To put it simply, a tire is a flexible rubber casing made to fit the rim of your vehicle’s wheel. However, the structure is a bit more complicated than that. There is also a number of required materials to make a tire you can actually rely on.
The primary raw material needed in tire manufacturing is rubber. The industry produces natural and synthetic rubbers on a wide scale. Due to the huge demand for tires, there is a shortage of natural rubber all around the world. It became inevitable to produce increasing amounts of rubber artificially, resulting in synthetic rubber.
Carbon black is also an essential ingredient, used in a fine powder form. Tire factories use a lot of this fine black fluffy particle, which is why they store it in big silos. Other ingredients include various chemicals. Each of them improves certain performance characteristics depending on the type of tire they want to produce.
While the end product is a one-fourth synthetic rubber tire, it also contains polyester, steel, nylon, silica, pigments, waxes, and reinforcing chemicals. Below, we are going to show you how exactly they make rubber, both natural and synthetic.
Back in the day when people started to produce rubber tires, there was no rubber tree plant shortage. They produced latex rubber from the liquid sap they extracted from these trees. Kundzu, oak trees, and poplars are the main sources of natural rubber.
They produce a volatile hydrocarbon liquid called isoprene, which forms the basic structural unit of the rubber. Cutting the bark and collecting the sap in a cup through a process called latex dripping. Then, they added ammonia to make sure the sap won’t solidify over time.
Next, they extracted the rubber by adding acid to the mix. After 12 hours, the resulting rubber compound was still wet, so they had to pass them through rollers and then hang them over racks. As the last step, they fold it into bales so that they can use them for tire production.
Thanks to chemistry, processing rubber is easier than ever in the tire-making industry.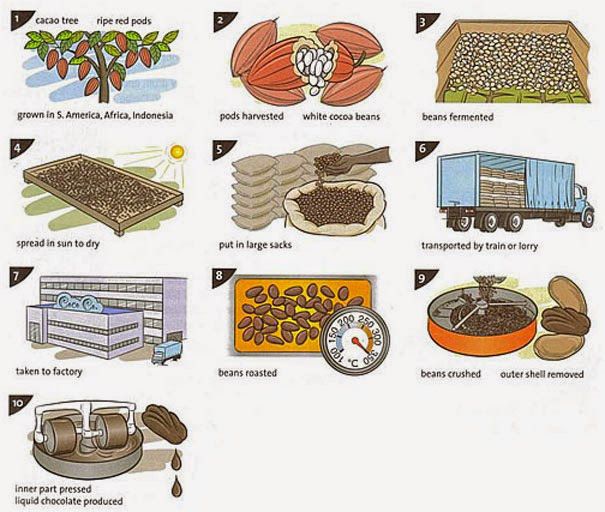 It is a great alternative to natural rubber, although its production requires lots of crude oil. This artificial rubber is made by linking polymer molecules together.
It is a great alternative to natural rubber, although its production requires lots of crude oil. This artificial rubber is made by linking polymer molecules together.
This is done in a laboratory called a chemical plant. The main ingredients are petrochemicals such as neoprene, which is made of hydrochloric acid and acetylene. Emulsion styrene-butadiene rubber or E-SBR is another great product of polymer chemistry.
It is one of the most commonly used general-purpose synthetic rubbers in the World. This rubber is the copolymer of styrene (25%) and butadiene (75%). To put it simply, the molecules of these two monomers are cross-linked through the vulcanization process, resulting in a strong structure.
Isoprene can be obtained by processing petroleum oil, resulting in pretty much the same volatile liquid hydrocarbon produced by trees. By combining isobutylene and isoprene, manufacturers can make butyl rubber as well. This is another type of synthetic rubber that comes with minimal gas and moisture permeability and outstanding shock absorption properties.
Carbon black is basically the main reason why we have black tires instead of white ones. It not only gives the tire its color but also helps with heat dissipation. It is a filler material that is produced at high temperatures.
They use combustion air, which is a source of oxygen to burn fuel, and burn it together with hydrocarbon fuel, which is oil or gas. The result is a chemical material in the form of a soft powder, as it is best to be used in small granules in tire making.
It is used to strengthen the tire’s structure and improve its certain characteristics. It protects against ozone and UV rays, boosts its abrasion resistance, and enhances tensile strength.
Now we finally know what is rubber made out of. We prepared the raw materials, but one question is still there: How are tires made? Well, tire manufacturers follow a strict protocol when it comes to tire assembly. We are still a couple of steps away from getting all tire components ready.
There are various types of tires in the market, yet they all go through a similar construction process.
At this stage, they mix together all different raw materials. The rubber polymers and additives constitute a mix that varies in consistency throughout the various parts of the tire. The reason for this is simple. You want to emphasize versatile characteristics for passenger tires than for industrial tires.
There is a huge difference between winter and summer car tires as well. The rubber is not exactly the same, which is why manufacturers adjust their recipes accordingly.
The next part of the manufacturing process rubberizes the components that reinforce the tire. These include cables, steel belts, and textiles for the most part. Depending on the type of tire in the making, manufacturers use 10 to 30 different components for a single tire.
Even passenger tires need steel cord belt plies, jointless cap plies (nylon embedded in rubber), textile cord plies, an inner liner of butyl rubber, etc.
They assemble all the abovementioned components using various machinery, resulting in “green tires”. At this stage, the tire gets its strong structure, although it still lacks the tread pattern. Assembly machines have their own belt drum where all components need to be placed.
It is where the tire gets its shape through stretching and pressing as all different parts come together.
The rubber has to go through a process called vulcanization, which means that part of the process treats it with high heat. You can imagine this part as just throwing it in the oven for some time to cook it. It is the heat treatment phase that solidifies the structure.
Manufacturers usually do this part with sulfur, resulting in the molecules cross-linking with each other and forming a stronger bond. The heat makes the rubber compounds stronger and more durable. Once it is “taken out of the oven” it is ready for shaping, depending on the tread pattern and overall tire design.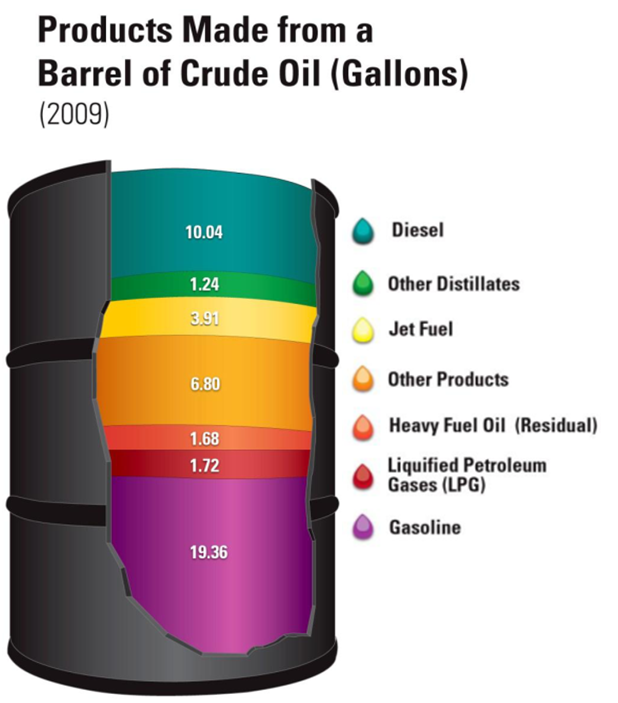
We need about five gallons of oil to produce the synthetic rubbers required for a single tire. The whole tire manufacturing process that follows requires two additional gallons of oil. The manufacturing process uses it to fuel the energy required to prepare the materials and assemble the whole tire.
Bigger ones such as truck tires require, even more, averaging 22 gallons of oil. The tire industry often uses gasoline, the production of which also requires oil. For every barrel of oil (42 gallons), they can produce about 19 gallons of gasoline.
The thing is that by making tires from natural rubber compounds instead of artificial ones, we do not take oil out of the whole equation. We still need it to produce the special additives needed to enhance the tire’s performance characteristics.
Moreover, we also need petroleum oil to work the tires into shape. Carbon black, one of the most essential ingredients is also a petroleum byproduct. It is produced through combustion, requiring various petroleum oil-based products in the process.
It is produced through combustion, requiring various petroleum oil-based products in the process.
Tire manufacturers have already found a way to produce carbon black without burning oil. They have found ways to produce it from plant-based oil, methane, and carbon dioxide. However, they still need to somewhat rely on the petroleum-based method.
As technology advances, we are inevitably going to reach the point when brands can manufacture tires in an environmentally-friendly way. Until then, we more or less need to use petroleum oil in the process.
We can get the rubber from natural sources such as rubber trees. The real problem here is that there is a shortage of trees available, compared to the global demand for tires. The global manufacturing of rubber products has already contributed to mass deforestation across the world.
There are simply not enough natural resources to get the desired amount of rubber produced. Fortunately, science has found a way to produce isoprene, the main ingredient of rubber without having to use oil in the process. A company called Genencor had a project with Goodyear during which they found a way to generate natural isoprene.
A company called Genencor had a project with Goodyear during which they found a way to generate natural isoprene.
They took plants such as corn cobs, switchgrass, and corn and used E. coli bacteria to break down their cellulose-based sugars. The result was natural isoprene and very few toxic waste products. This way, they have demonstrated an environmentally-friendly process.
They now call the end product BioIsoprene, which enables manufacturers to produce rubber compounds without using oil.
The reason why tires are black is quite simple. We have previously talked about what brands use to make rubber, and there is nothing black in it. The ingredient that makes tires black is carbon black, which brands add to the mix later in the tire manufacturing process. It is crucial for the tire’s abrasion resistance and tensile strength. Due to the resulting strengthened structure, this ingredient makes the tire last longer.
Without carbon black, tires made of natural rubber would be white in color. They would also have weak performance characteristics that would make them unsuitable for everyday use. It would result in short service life, wearing down tread patterns at a fast pace.
They would also have weak performance characteristics that would make them unsuitable for everyday use. It would result in short service life, wearing down tread patterns at a fast pace.
One of the biggest problems with tires is friction-induced pollution. You see, not all pollution comes from the tire manufacturing process. As you drive your car, your tires are constantly generating friction between the footprint and the road surface.
The result is plenty of tire dust that has a huge impact on the environment. As your tires grip the road, they dissipate energy and waste heat depending on their rolling resistance. When we do the math, it turns out that 9 percent of oil consumption is the result of tire rolling resistance. Low rolling resistance means less friction, less pollution, and fuel efficiency.
By making tires, tire manufacturers contribute to pollution by only 1 percent. We can only get that oil through oil refining, which generates hazardous wastes and pollutes our air and water as well. The devil is in the details, as they say.
The devil is in the details, as they say.
Brands make most tires using synthetic rubber. On average, to produce one tire, the process uses seven gallons of oil. Tires are not directly and solely made of oil, it is an essential ingredient for synthetic rubber. Even when using natural rubber, tire manufacturing needs oil later in the process.
The industry makes rubber from both natural ingredients and artificial ones. Natural rubber comes from rubber trees, extracting latex from the tree – a liquid sap produced by the tree. Besides latex rubber, there is synthetic rubber, made with petrochemical feedstocks through chemical processes.
Plastic and rubber fall under the category of polymers. They have to mix additives with polymers in order to gain additional properties. Polymers with an elastic property are elastomers. They differently arrange polymer molecules in rubbers, enabling them to bend and return to their initial shape.
Media Platforms Design Team
The field of transportation is ripe for green tech: It accounts for two-thirds of U.S. oil consumption and generates one-third of the nation's greenhouse gas emissions. Most research has focused on alternatives to gasoline-powered internal-combustion engines, but multinational biotechnology company Genencor is working on a more plebian car component: tires.
About 250 million tires are sold yearly in the United States. Each one is roughly one-fourth synthetic rubber (the rest consists of natural rubber, steel, nylon, polyester, assorted reinforcing chemicals, waxes, pigments and oils). Synthetic rubber production dates back to the early 1900s and mushroomed into an industry during World War II. Today it takes about seven gallons of oil to make a standard tire—five gallons as feedstock for chemicals that make up synthetic rubber, plus two for the energy required to power the manufacturing process.
Many types of plants, including poplars, oak trees and kudzu, produce isoprene, a volatile hydrocarbon liquid that's a building block of natural rubber. (Isoprene emissions from trees on hot days are a component of smog.) Chemical companies also refine isoprene from petroleum and use it to make synthetic polyisoprene rubber, which has good strength, flexibility and resistance to cold. Synthetic polyisoprene is widely used in tires, as well as other goods like hoses, rubber bands and pipe gaskets.
Working with Goodyear Tire & Rubber, Genencor has developed a way to make isoprene by starting with plant material instead of oil. The company uses E. coli bacteria to break down cellulose-based sugars derived from plant materials like corn, corn cobs or switchgrass, generating natural isoprene as a byproduct.
It's cleaner to make than the oil version. "Some of the microbe cell mass is left over after the microbes catalyze the reaction," says Genencor vice president Carl Sanford, "but that can be recycled, used as fertilizer or burned for energy. There are no highly toxic waste products."
There are no highly toxic waste products."
Genencor delivered the first shipment of its BioIsoprene™ to Goodyear early last year, and it was incorporated into two concept tires displayed at the Copenhagen climate change conference in December. In March Genencor announced that it had inserted plant genes for producing isoprene into E. coli bacteria, speeding up the manufacturing process. The company plans to start pilot production of BioIsoprene within two years, and says that bio-based tires could be on the market within three to five years.
Genencor's business case relies on making BioIsoprene cost-competitive with isoprene from petroleum, so its prospects hinge partly on world oil prices over the next five years. Global rubber supplies will also be a factor. BioIsoprene could sub in for a portion of the natural rubber already in tires, Sanford says. "And sometimes companies can't get enough petro-derived isoprene, so we may be able to fill the void. "
"
Genencor will no doubt have competition for the plant feedstock: Under the Environmental Protection Agency's Renewable Fuel Standard program, first enacted in 2005 and amended several times since, U.S. oil refiners are required to blend 3 billion gallons of cellulosic biofuels into gasoline annually by 2015, and 16 billion gallons per year by 2022. While federal agencies have estimated that the U.S. could produce as much as a billion tons of biomass crops per year within the next several decades, enough to satisfy the renewable fuel mandate with materials left over, not all experts agree on that number.
It's worth noting, however, that although the global isoprene market isn't trivial—about 1.7 billion pounds per year—replacing that entire amount with plant-based isoprene is a much smaller undertaking than meeting federal biofuel targets. Sanford estimates that every ton of BioIsoprene requires about four tons of feedstock, so it would take only about 3. 4 million tons of cellulosic materials to make enough plant-based isoprene for all global applications.
4 million tons of cellulosic materials to make enough plant-based isoprene for all global applications.
According to a life-cycle assessment by European tire and rubber manufacturers, the biggest environmental impacts from tires occur not while they're being made, but while they're being used. The main culprit is rolling resistance (friction and other forces that are generated as tires grip the road and dissipate energy as waste heat). Michelin, the world's largest tire manufacturer, estimates that rolling resistance accounts for 9 percent of global oil consumption; in contrast, making tires represents less than 1 percent. Low rolling resistance tires, which are widely available now, can improve drivers' gas mileage by 3 to 4 percent.
"There are three things we must do to move toward a sustainable technology base," says Terry Collins, professor of chemistry and director of the Institute for Green Science at Carnegie Mellon University.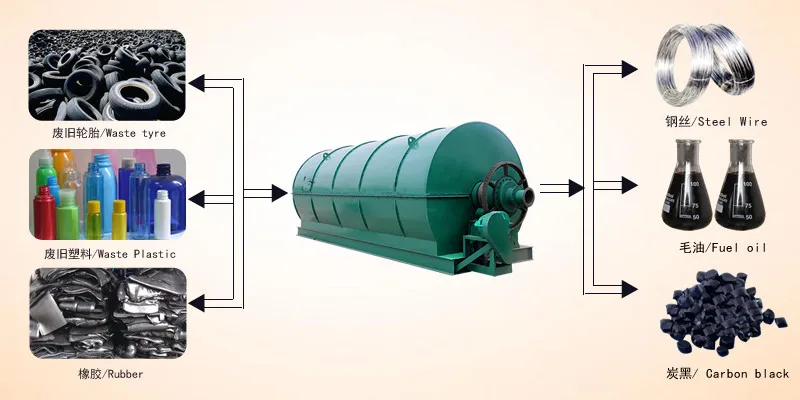 "Go solar for energy, move from fossilized to recently dead plant matter for the source materials of our products and reduce and eliminate toxic substances from our technologies." Because oil refining generates high levels of air and water pollution and hazardous wastes, reducing the need for petroleum-based inputs would be reason to call BioIsoprene a green product.
"Go solar for energy, move from fossilized to recently dead plant matter for the source materials of our products and reduce and eliminate toxic substances from our technologies." Because oil refining generates high levels of air and water pollution and hazardous wastes, reducing the need for petroleum-based inputs would be reason to call BioIsoprene a green product.
The final verdict, says Collins, depends on what kinds of energy, water and other resources are required to manufacture it commercially. "The devil, and the angels, are in the details," he says.
Hello guys. How much oil should be poured into the tire on such a mower?
Oil Tip Mower
Share
Hello everyone. Does anyone know why oil is running from the mower to the lower cup? Chanterelle mower. Thank you.
Does anyone know why oil is running from the mower to the lower cup? Chanterelle mower. Thank you.
Why Oil is flowing Oil Cup lower Chanterelle mower
Mower Rotary mower Polish Z-178 What is better Fill in the trough Bathtub Lubrication Oil Mining Recycling
Good luck to everyone. Please tell me. Why does engine oil get into the radiator of a Yumz tractor?
Why Engine Oil Gets Radiator Tractor YuMZ
Can the MTZ 82 tank be installed?
Hello. Please tell me, oil flows between the stove and the block, how to fix it?
Please tell me, oil flows between the stove and the block, how to fix it?
Why Oil Flows between Stove Block How to fix
Guys, such a question, do I need to remove the belts for the winter on the Chanterelle mower? How do you prepare your equipment for the winter? Admin please post
Is it necessary? Remove winter Belts Mower fox
 I checked the valve on the distribution, reduced the pressure, it didn’t help, and the oil is also heated in the hydraulics, as if one section is turned on. What the hell don't I understand?
I checked the valve on the distribution, reduced the pressure, it didn’t help, and the oil is also heated in the hydraulics, as if one section is turned on. What the hell don't I understand? installed Distributor rp 70 MTZ 80 Why does the engine work when starting Load Oil Hydraulics Heats up
Hello everyone. Mtz 82 has a problem with hydraulics, it lifts everything perfectly when it is cold, as the oil heats up everything is so, they put a zero pump, the same thing. Even with this new pump, as the oil heats up, it starts to work loudly as you press the lever. I understand there is no oil coming in. Or could there be something else? Thank you!
МТЗ 82 Problem Hydraulics Why On a cold Raises Oil Heats up no Zero Pump set the same
 what to do?
what to do? Good day. Tell me the engine d260 oil from the engine went into the cooling system. Where to flatter? Thanks in advance, colleagues!
Engine D-260 Oil Engine gone Cooling system What to do
Guys, tell me, please, after changing the head to MTZ 80, it throws oil from the muffler. What is the reason?
Why After replacement, the MTZ 80 head is thrown out Oil Silencer
One of the duties of the driver, prescribed in the owner's manual, is to monitor the oil level in the engine. What to do if the level falls below the minimum: how urgently do you need to top up, which one? The answers to these and other frequently asked questions are in our article.
A normal oil level is essential for the most effective wear protection of parts. To control the level in the engines, a dipstick is provided, which is easily accessible from the engine compartment. The check is carried out visually. Min and Max marks are marked on the probe (usually the space between them is made with a plastic nozzle, corrugation or other methods). When the dipstick is removed, the oil should be between these marks.
On relatively new vehicles, the oil level is always within acceptable limits. There is no need to top it up: just call in for a timely replacement. Specialists of the Favorit Motors Group of Companies remind that each vehicle has its own frequency: for example, for European models with gasoline engines, it is 15,000 km or (under severe vehicle operating conditions) 10,000 km. The exact service interval can be found in the instruction manual. The need for replacement is due to the fact that the oil loses its properties: additives run out of life, the smallest wear products accumulate, which the filter cannot hold. Even if you rarely drive a car, you should change the oil once a year.
Even if you rarely drive a car, you should change the oil once a year.
We are used to the fact that in all cars there are indicators on the instrument panel with the image of an oiler or the inscription OIL. Many drivers do not bother to check the oil level, hoping for the help of the on-board diagnostic system. But this is not always justified. The fact is that the same indicator indicates a problem with oil pressure, and not its level. In simple terms, the oil pump takes oil from almost the very bottom of the sump. Accordingly, while it is, in principle, there will be no problems with pressure in normal modes. They can occur during sudden maneuvers, driving uphill or downhill, and only then air enters the pump and the light comes on. So relying on a familiar indicator in terms of level control is wrong.
In fairness, we note that in some cars, during self-diagnosis, it is checked, including the amount of oil. This makes life much easier for the driver.
Although such a check is an elementary procedure, there are several fundamentally important rules for its implementation. First, control is best done on a cold engine. In this case, all the oil is in the sump - during the trip, it is pumped by a pump and sprayed throughout the motor. If you perform a "hot engine" check, the level may appear higher than it really is. Secondly, it is advisable to remove the dipstick before assessing the level, wipe it, then carefully immerse it back and remove it again. Otherwise, the level is not always correctly "read" on the dipstick.
In heavily worn engines, grease leaks through leaky seals. Also, the oil is consumed "for waste", that is, it burns out in the engine cylinders. The more worn the oil rings on the pistons, the more oil will be lost. Modern engines sometimes consume a fairly large volume and this is stated in the instructions: for example, in German cars, oil consumption up to 1 liter per 1000 km is considered the norm.
If you see that the oil level is below normal, it must be topped up as soon as possible, otherwise the power unit will experience oil starvation and wear out intensively. Ideally, add the same oil that is already filled in your internal combustion engine. For those who are serviced at Favorit Motors Group dealerships, we advise you to look for lubricants on our website - here you can buy containers of both 1 liter and 4-5 liters.
Why is it not recommended to use other oils, even from the same manufacturer? Each type of oil uses its own additives, which are not always compatible with others. As a result, after topping up, a precipitate may form, turbidity, viscosity change - in a word, the oil mixture will have different characteristics.
If you are far from your service and cannot find what you need, follow these rules. It is permissible to add another to mineral oil, but on a mineral basis. Similarly with synthetics: it is better to use synthetic. Semi-synthetic oils are universal: they can be mixed with any others, and any other can be added to “semi-synthetics”. Try to add oil to the minimum allowable level in order to buy “native” oil if possible and fill it to the full volume.
Semi-synthetic oils are universal: they can be mixed with any others, and any other can be added to “semi-synthetics”. Try to add oil to the minimum allowable level in order to buy “native” oil if possible and fill it to the full volume.
In the most extreme case, when there is no oil, but you have to go, you can add any oil to any oil. Here we actually choose between two evils: driving without oil is much worse. When driving, try not to load the engine unnecessarily, do not spin it up to high speed. Upon return, the resulting "motor fluid" should be replaced with normal oil, preferably with a flush.
You need to fill in the oil through a funnel or from the neck of the canister in portions of 200-300 grams, wait a few minutes until it reaches the crankcase from the filler neck and only then check the level.
If the engine consumes engine oil quite actively, a logical question arises: is it possible to pour it “with a margin” so as not to get under the hood so often? No you can not. With excess oil, it will be squeezed out through all the gaskets, in addition, there is a risk of squeezing out the crankshaft oil seals. In winter, the oil thickens, and the more it is in the motor, the more difficult it is to turn the shaft to start. Therefore, overflow is not allowed.
With excess oil, it will be squeezed out through all the gaskets, in addition, there is a risk of squeezing out the crankshaft oil seals. In winter, the oil thickens, and the more it is in the motor, the more difficult it is to turn the shaft to start. Therefore, overflow is not allowed.
Another popular question. The logic is this: if you periodically top up the oil, that is, renew it, it should last longer. But it is not so. Products of combustion and wear of parts accumulate in the oil - not all are retained by the oil filter. That is why the initially translucent oil darkens after the first thousand kilometers. When oil burns or leaks through gaskets and seals, wear and combustion products remain inside. You can get rid of them only with a complete oil change. If you add 1 liter of oil and then another 1 liter, it seems to you that you have already replaced 2 liters out of, say, 4. But this is not so: after all, the first liter was mixed with the “dirty” contents of the lubrication system.